Research
COVID-19 Modelling
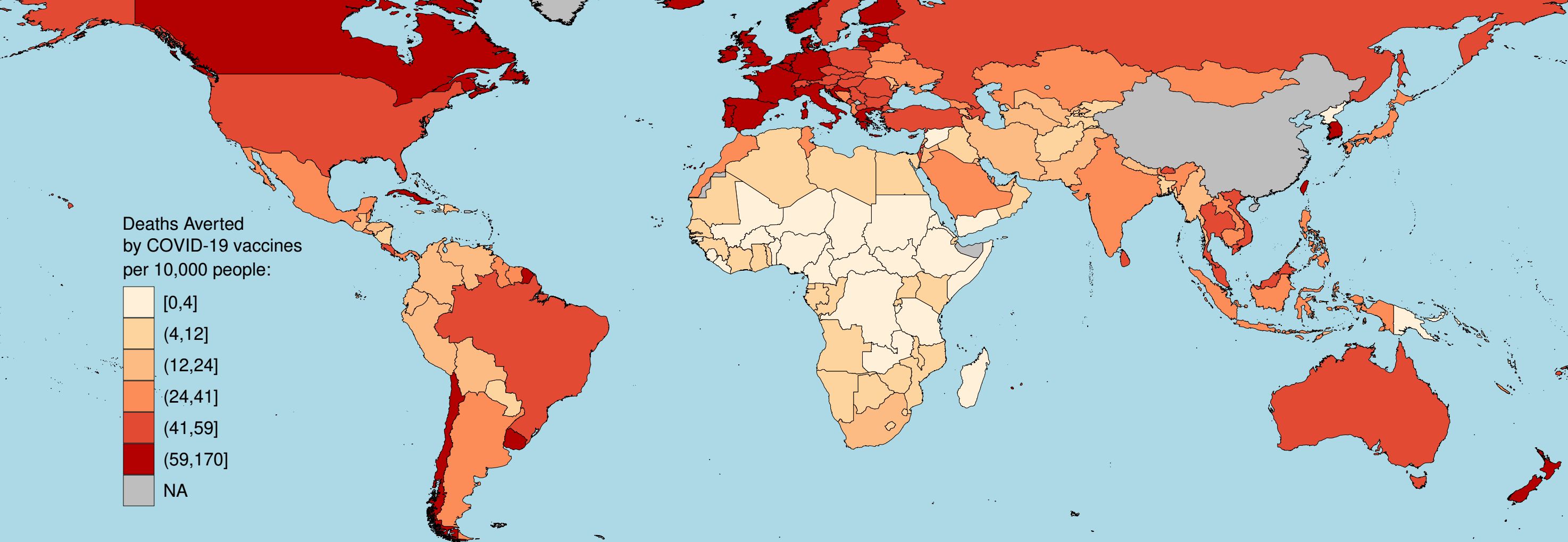
In March 2020, I joined the Imperial College COVID-19 response team. I led the software development and modelling in low- and middle-income countries (LMICs), producing epidemic trajectories for the potential global burden of COVID-19 (Science). Throughout the pandemic, I provided epidemic scenario projections (Imperial College COVID-19 LMIC Reports) for every country to the WHO as part of the first costing for a global response to COVID-19 (Lancet Global Health) and the WHO Essential Supplies Forecasting Tool. Most recently, in June 2022, I published the first estimates of the global impact of the COVID-19 vaccinations, showing almost 20 million deaths were averted due to vaccination during the first of vaccinations (Lancet Infectious Diseases).

Generating Novel Mortality Data Sources in low-income settings
As part of growing evidence of COVID-19 mortality being unobserved, I led two research studies in Damascus (Nature Communications) and Khartoum (Imperial College COVID-19 Report 39) to ascertain mortality reporting of COVID-19. In Damascus, I identified Facebook groups uploading obituary certificates since the start of the conflict in Damascus. These obituary certificates provided a reliable source of mortality patterns in the city that revealed clear COVID-19 epidemic dynamics. In Khartoum, I worked with local youth activist groups to integrate surveys of COVID-19 symptoms distributed through social media channels to estimate the proportion of COVID-19 deaths occurring undetected. The latter was part of evidence that led to new ministry of health intervention measures and increased public health campaigns to raise awareness of COVID-19 during Sudan’s second wave.

Demographic and Health Surveys Program (DHS) Software:
I have developed an open source software package, rdhs, that is the first non-proprietary tool to ease access to and analysis of the DHS datasets. The software was developed to address the need for greater democratization of lower-middle income country data and was presented at the ASTMH annual meeting in 2018. The package has been downloaded over 23,000 times since it was published in December 2018.
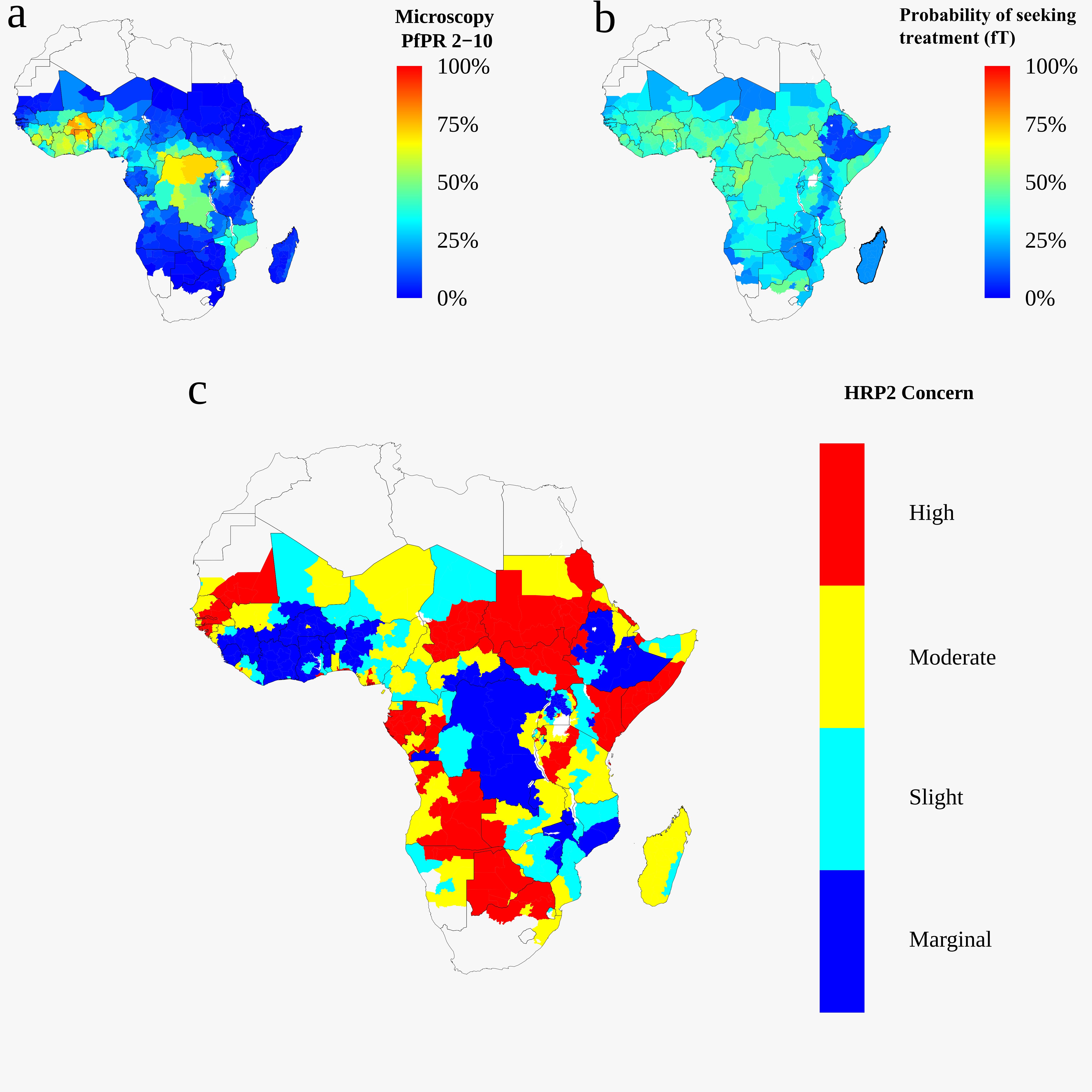
Malaria transmission modelling:
In 2016, a study in the Democratic Republic of Congo provided evidence that malaria parasites were evading detection by rapid diagnostic tests due to the deletion of the pfhrp2 gene. Using mathematical transmission models, I provided evidence that there was selection of pfhrp2 gene deletions to a WHO panel steered by the director of the WHO Global Malaria Programme. The panel formulated WHO guidance to national malaria control programmes (NMCPs) to implement molecular surveys to estimate pfhrp2 deletion prevalence. After attending the Malaria Policy Advisory Committee meeting in November 2017, I conducted a collaborative study with the WHO to estimate the potential for seasonal variations in malaria transmission to introduce bias in estimating pfhrp2 deletion prevalence. These findings infomed NMCPs through an interactive online database and field training programmes to optimise surveillance for pfhrp2. (Both studies in eLife). Additionally, I have modelled the impact of false-negative RDTs on community surveys (BMJ Global Health), worked on modelling ivermectin as a possible addition to the currently available malaria control tools (Lancet Infectious Diseases), as well as a modelling-based evaluation of the utility of malaria genetics for inferring transmission intensity (Molecular, Biology and Evolution) and the impact of partner drug resistance on artemisinin resistance (Lancet Microbe).